Laboratory Research
(LR-011) Radiation injury triggers a cutaneous scarring response by inducing expression of pro-fibrotic genes and increasing collagen thickness in an ex vivo human skin model
Friday, April 28, 2023
7:15 PM - 8:30 PM East Coast USA Time
Lindsey Siegfried, MS – Department of Dermatology and Cutaneous Surgery – University of Miami Miller School of Medicine, Miami, FL, United States; Irena Pastar, PhD – Department of Dermatology and Cutaneous Surgery – University of Miami Miller School of Medicine, Miami, FL, United States; Seth Thaller, MD – Division of Plastic, Aesthetic, and Reconstructive Surgery – University of Miami Miller School of Medicine, Miami, FL, United States; Rivka Stone, MD, PhD – Department of Dermatology and Cutaneous Surgery – University of Miami Miller School of Medicine, Miami, FL, United States
Introduction: Radiation-induced skin fibrosis (RISF) is a devastating outcome of cutaneous injury by irradiation that is delivered as part of clinical care, causing impaired mobility and function, disfigurement, and diminished quality of life for patients. Though animal models for RISF have been developed, experimental models of evolving RISF in human skin are needed in order to identify biomarkers that reflect the development, progression, and severity of RISF in patients.
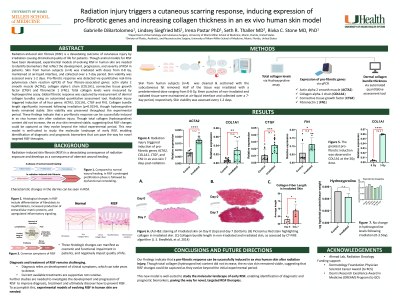
Methods: To model RISF, skin from human subjects (n=4) was irradiated with doses ranging from 0-8 Gy, maintained at air-liquid interface, and collected over a 7-day period. Skin viability was assessed every 1-2 days. Pro-fibrotic response was detected via quantitative real-time PCR of four fibrosis-associated genes: actin alpha 2 smooth muscle (ACTA2), collagen alpha-1 chain (COL1A1), connective tissue growth factor (CTGF) and fibronectin 1 (FN1). Total collagen levels were measured by hydroxyproline assay. Global fibrotic response was captured by measurement of dermal collagen bundle thickness using an automated quantitative assessment tool.
Results: Radiation injury triggered induction of all four pro-fibrotic genes: ACTA2 (2.2-fold increase; p< 0.0001); COL1A1 (5.9-fold increase; p=0.003), CTGF (4.1-fold increase; p< 0.0001), and FN1 (3.8-fold increase; p< 0.0001). The greatest pro-fibrotic induction was observed at the 3Gy dose, with a 12.2-fold induction in COL1A1 7 days post-treatment. Collagen bundle length and width significantly increased following irradiation, though hydroxyproline levels remained stable. Skin viability was preserved throughout the experimental period.
Discussion: A pro-fibrotic response was successfully induced in ex vivo human skin after radiation injury, as evidenced by significant upregulation of four key fibrosis-associated genes, as well as by increased collagen bundle thickness on histologic sections. Though total collagen (hydroxyproline) content did not increase, the ex vivo skin remained viable, suggesting that RISF changes could be captured as they evolve beyond the initial experimental period. Taken together, this new model is well-suited for study of the molecular landscape of early RISF, enabling identification of diagnostic and prognostic biomarkers that can pave the way for novel targeted RISF therapies.
Methods: To model RISF, skin from human subjects (n=4) was irradiated with doses ranging from 0-8 Gy, maintained at air-liquid interface, and collected over a 7-day period. Skin viability was assessed every 1-2 days. Pro-fibrotic response was detected via quantitative real-time PCR of four fibrosis-associated genes: actin alpha 2 smooth muscle (ACTA2), collagen alpha-1 chain (COL1A1), connective tissue growth factor (CTGF) and fibronectin 1 (FN1). Total collagen levels were measured by hydroxyproline assay. Global fibrotic response was captured by measurement of dermal collagen bundle thickness using an automated quantitative assessment tool.
Results: Radiation injury triggered induction of all four pro-fibrotic genes: ACTA2 (2.2-fold increase; p< 0.0001); COL1A1 (5.9-fold increase; p=0.003), CTGF (4.1-fold increase; p< 0.0001), and FN1 (3.8-fold increase; p< 0.0001). The greatest pro-fibrotic induction was observed at the 3Gy dose, with a 12.2-fold induction in COL1A1 7 days post-treatment. Collagen bundle length and width significantly increased following irradiation, though hydroxyproline levels remained stable. Skin viability was preserved throughout the experimental period.
Discussion: A pro-fibrotic response was successfully induced in ex vivo human skin after radiation injury, as evidenced by significant upregulation of four key fibrosis-associated genes, as well as by increased collagen bundle thickness on histologic sections. Though total collagen (hydroxyproline) content did not increase, the ex vivo skin remained viable, suggesting that RISF changes could be captured as they evolve beyond the initial experimental period. Taken together, this new model is well-suited for study of the molecular landscape of early RISF, enabling identification of diagnostic and prognostic biomarkers that can pave the way for novel targeted RISF therapies.

.png)