Laboratory Research
(LR-018) A Novel In Vitro Assay for Evaluating the Malodor Inhibition Properties of Wound Dressings Containing Odor Abatement Technologies
Friday, April 28, 2023
7:15 PM - 8:30 PM East Coast USA Time
Patrick Lamb, MS, MBA – VP International Operations, Biodaptive, LLC
Introduction: Offensive odor emanating from a wound is indicative of bacterial colonization and/or necrotic tissue within the wound. 1,2,3 While malodor itself is not harmful to patients, it can be unpleasant and distracting to the patient, relatives, and caregivers. Consequently, a number of wound dressings are commercially available that help to manage the wound environment to promote healing and contain odor abatement (OA) technologies to mitigate malodors. These technologies act by either killing the bacteria that cause the malodor using antimicrobial agents or they adsorb/capture the volatile compounds responsible for the malodor. The mode-of-action by which odor reduction occurs can significantly impact how the FDA classifies the device, therefore being able to differentiate the MOA is critical. The novel in vitro assay utilized in this study was designed to quantitatively demonstrate odor reduction and differentiate the MOA of the OA technology.
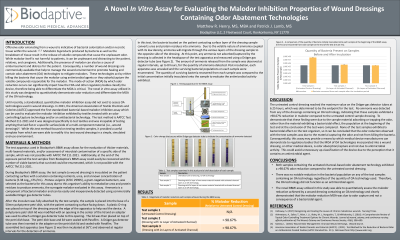
Methods: Wound dressings containing an OA technology and untreated controls were evaluated in vitro using a bacterial based malodor inhibition (BBMI) assay. During this test, the patient contacting surface of the dressings were inoculated with a solution containing nutrients, urea and a known concentration of bacteria (5.38 Log10CFU/mL). Proteus vulgaris was selected as the bacteria due to this organism’s ability to metabolize urea to produce ammonia, the surrogate malodor evaluated in this assay. Samples were incubated at 36°C for 6.25 hours in a specially designed apparatus. Any ammonia not absorbed/captured as it migrated through the dressing was collected and measured using a Dräger gas detector tube. Post-incubation, each apparatus was unsealed and the surviving bacterial concentration on each sample was enumerated.
Results: The wound dressings containing an OA technology exhibited a 99% reduction in malodor over a 6.25 hour time point compared to the untreated control sample. Additionally, neither the test samples nor control samples exhibited a bactericidal effect during the test.
Discussion: The novel BBMI assay utilized in this study was able to quantitatively assess the malodor reduction achieved by a wound dressing containing an OA technology and clearly demonstrated that the malodor reduction MOA was due to odor capture and not a consequence of a bactericidal agent.
Methods: Wound dressings containing an OA technology and untreated controls were evaluated in vitro using a bacterial based malodor inhibition (BBMI) assay. During this test, the patient contacting surface of the dressings were inoculated with a solution containing nutrients, urea and a known concentration of bacteria (5.38 Log10CFU/mL). Proteus vulgaris was selected as the bacteria due to this organism’s ability to metabolize urea to produce ammonia, the surrogate malodor evaluated in this assay. Samples were incubated at 36°C for 6.25 hours in a specially designed apparatus. Any ammonia not absorbed/captured as it migrated through the dressing was collected and measured using a Dräger gas detector tube. Post-incubation, each apparatus was unsealed and the surviving bacterial concentration on each sample was enumerated.
Results: The wound dressings containing an OA technology exhibited a 99% reduction in malodor over a 6.25 hour time point compared to the untreated control sample. Additionally, neither the test samples nor control samples exhibited a bactericidal effect during the test.
Discussion: The novel BBMI assay utilized in this study was able to quantitatively assess the malodor reduction achieved by a wound dressing containing an OA technology and clearly demonstrated that the malodor reduction MOA was due to odor capture and not a consequence of a bactericidal agent.

.png)