Laboratory Research
(LR-001) The formulation of biofilm using accredited CDC model and qPCR
Friday, April 28, 2023
7:15 PM - 8:30 PM East Coast USA Time
Laura Sellars, PhD; Samantha Westgate, PhD
Introduction: The CDC Biofilm Reactor® is widely used for commercial product testing of biofilm established onto solid state coupons. Bacterial quantification through standard microbiological techniques is mainly used in the assessment of biofilm removal or prevention. However, it widely known that bacteria alter gene expression profiles as environmental pressures evolve. A greater understanding of key genes in response to the presence of antimicrobial products will allow wound companies to understand further about the mode of action of their products in relation to biofilm prevention and biofilm removal.
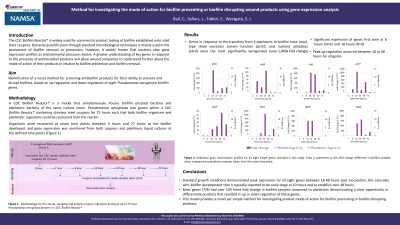
Methods: A Pseudomonas aeruginosa inoculum was prepared and transferred to a CDC Biofilm Reactor® containing stainless steel coupons. The reactor was incubated for 72 hours at 37oC under shaking conditions.
Both biofilm bacteria attached to the coupons and planktonic bacteria were recovered at six time points between 2 hours and 72 hours as the biofilm developed, and gene expression was monitored from both coupons
Results: All investigated genes were significantly up‑regulated by 18 hours, with four (pelA, rsaL, pcrV and gyrA) peaking at 18 hours, and the remaining four (pslA, psqC, acpP and cbrA) peaking at 24 hours.
Discussion: Results showed that the peak expression for all eight genes under typical P. aeruginosa biofilm formation occurred between 18-48 hours post inoculation, which coincides with biofilm development. In addition, despite a similar bacterial load, most genes had over 100 times fold change in biofilm samples compared to planktonic, providing the framework for analysing mode of action for wound care products targeting biofilms. This novel method would be recommended for screening antibiofilm products for their ability to prevent and disrupt biofilms.
Methods: A Pseudomonas aeruginosa inoculum was prepared and transferred to a CDC Biofilm Reactor® containing stainless steel coupons. The reactor was incubated for 72 hours at 37oC under shaking conditions.
Both biofilm bacteria attached to the coupons and planktonic bacteria were recovered at six time points between 2 hours and 72 hours as the biofilm developed, and gene expression was monitored from both coupons
Results: All investigated genes were significantly up‑regulated by 18 hours, with four (pelA, rsaL, pcrV and gyrA) peaking at 18 hours, and the remaining four (pslA, psqC, acpP and cbrA) peaking at 24 hours.
Discussion: Results showed that the peak expression for all eight genes under typical P. aeruginosa biofilm formation occurred between 18-48 hours post inoculation, which coincides with biofilm development. In addition, despite a similar bacterial load, most genes had over 100 times fold change in biofilm samples compared to planktonic, providing the framework for analysing mode of action for wound care products targeting biofilms. This novel method would be recommended for screening antibiofilm products for their ability to prevent and disrupt biofilms.

.png)