Clinical Research
(CR-016) Comparative Effectiveness Analyses for Medicare Beneficiaries with Diabetic Foot Ulcers Treated With and Without Dehydrated Amnion/Chorion Membrane (dACM)
Friday, April 28, 2023
7:15 PM - 8:30 PM East Coast USA Time
Bradford Rice, PhD; Jian-Yu e, ScD; Serena Kongara, MPH; Justin Chun, MHS; Robert Kirsner, MD, PhD
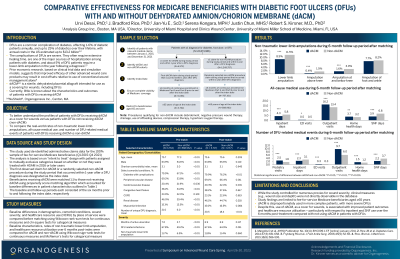
Introduction: Dehydrated amnion/chorion membrane (dACM) is intended for use as a placental allograft wound covering for the management of acute and chronic wounds including diabetic foot ulcers (DFUs). However, there is limited information about the real-world clinical and economic outcomes associated with using dACM* for people with DFUs.
Methods: The 100% Medicare Fee-For-Service data in Standard Analytic File (Q1 2015-Q4 2021) were used to identify two mutually-exclusive cohorts of beneficiaries with DFUs: (i) beneficiaries who received dACM* (first claim was considered as the index date), and (ii) beneficiaries who received advanced wound care (e.g., debridement, offloading, negative pressure wound therapy) but not dACM* (index date selected at random). Beneficiaries were required to have continuous enrollment in Medicare Parts A and B at least 6 months before and after the index date. Patients receiving dACM* were matched 1:1 to those not receiving dACM* using propensity score matching. Outcomes over 6 months post-index were compared between matched cohorts using statistical tests for paired data. Standardized difference (SD) >10% and p-values < 0.05 were considered statistically significant.
Results: Among all beneficiaries meeting the selection criteria, 3,762 received dACM* and 589,962 did not receive dACM*. Compared to beneficiaries not receiving dACM*, those receiving dACM* had a greater disease severity before treatment initiation as indicated by longer duration of active ulceration (7.0 vs. 3.7 months), higher rates of DFU-related infections (67.8% vs. 49.2%), non-traumatic lower-limb amputations (11.7% vs. 6.9%), and higher total medical costs ($36,118.7 vs. $24.126.0). During a 6-month follow-up period, matched beneficiaries receiving dACM* (N=3,717) had lower rates of non-traumatic lower-limb amputations (8.9% vs. 11.5%; SD = -8.62%; p < 0.001), as well as shorter stays and lower costs for hospitalization (4.3 vs. 5.8 days; $9,543.4 vs. $12,537.3) and skilled nursing facility (3.9 vs. 6.8 days; $1,650.3 vs. $2,647.1; SD >10% and p < 0.001 for all comparisons) than similar beneficiaries not receiving dACM*.
Discussion: The study suggests that dACM* is disproportionately used to treat more complex beneficiaries, with more severe DFUs. After adjusting for baseline differences, beneficiaries receiving dACM* had lower rates of amputations, and lower costs for hospitalization and skilled nursing facility visits than those not receiving dACM*.
Methods: The 100% Medicare Fee-For-Service data in Standard Analytic File (Q1 2015-Q4 2021) were used to identify two mutually-exclusive cohorts of beneficiaries with DFUs: (i) beneficiaries who received dACM* (first claim was considered as the index date), and (ii) beneficiaries who received advanced wound care (e.g., debridement, offloading, negative pressure wound therapy) but not dACM* (index date selected at random). Beneficiaries were required to have continuous enrollment in Medicare Parts A and B at least 6 months before and after the index date. Patients receiving dACM* were matched 1:1 to those not receiving dACM* using propensity score matching. Outcomes over 6 months post-index were compared between matched cohorts using statistical tests for paired data. Standardized difference (SD) >10% and p-values < 0.05 were considered statistically significant.
Results: Among all beneficiaries meeting the selection criteria, 3,762 received dACM* and 589,962 did not receive dACM*. Compared to beneficiaries not receiving dACM*, those receiving dACM* had a greater disease severity before treatment initiation as indicated by longer duration of active ulceration (7.0 vs. 3.7 months), higher rates of DFU-related infections (67.8% vs. 49.2%), non-traumatic lower-limb amputations (11.7% vs. 6.9%), and higher total medical costs ($36,118.7 vs. $24.126.0). During a 6-month follow-up period, matched beneficiaries receiving dACM* (N=3,717) had lower rates of non-traumatic lower-limb amputations (8.9% vs. 11.5%; SD = -8.62%; p < 0.001), as well as shorter stays and lower costs for hospitalization (4.3 vs. 5.8 days; $9,543.4 vs. $12,537.3) and skilled nursing facility (3.9 vs. 6.8 days; $1,650.3 vs. $2,647.1; SD >10% and p < 0.001 for all comparisons) than similar beneficiaries not receiving dACM*.
Discussion: The study suggests that dACM* is disproportionately used to treat more complex beneficiaries, with more severe DFUs. After adjusting for baseline differences, beneficiaries receiving dACM* had lower rates of amputations, and lower costs for hospitalization and skilled nursing facility visits than those not receiving dACM*.

.png)